Factors controlling nanosized Ni–Al2O3 catalysts synthesized by solution combustion for slurry-phase CO methanation: the ratio of reducing valences to oxidizing valences in redox systems
Abstract
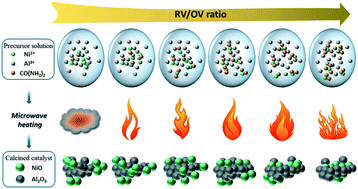
A series of nanosized Ni–Al2O3 catalysts for the catalytic methanation of CO were prepared by solution combustion of Ni2+ and Al3+ nitrates with urea. The main influence of the combustion process on the physicochemical and catalytic properties of Ni–Al2O3 catalysts were investigated by using the RV/OV ratio of reducing valences from urea to oxidizing valences from nitrates in redox systems. With increasing RV/OV ratio, more urea molecules coordinate with Ni2+ to form nickel ammine and enhance the diffusion of Ni2+ in the precursor solution. During the combustion process, the combustion enthalpy and gases increase with increasing RV/OV value, operating synergistically to achieve controlled physicochemical properties of the obtained catalysts. Especially, when RV/OV ≤ 0.75, the released gases are critical to the formation of a high surface area to disperse the NiO nanoparticles, whereas when RV/OV ≥ 0.75, the combustion enthalpy produces a high temperature, facilitating NiO migration into the Al2O3 matrix to form the low activity precursor NiAl2O4 spinel. The catalyst with RV/OV = 0.75 exhibits the maximum nickel surface area and the smallest Ni particle size of 62.6 m2 g−1 and 10.8 nm, respectively, which result in the optimum catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...