Effect of magnesium substitution into Fe-based La-hexaaluminates on the activity for CH4 catalytic combustion
Abstract
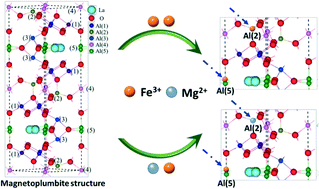
With the introduction of Mg2+ into Fe-substituted La-hexaaluminate precursors, a series of monophasic LaMgxFeAl11−xO19 (x = 0, 0.3, 0.7, 1) catalysts were prepared and tested as catalysts for CH4 catalytic combustion. The results show that the introduction of an appropriate amount of Mg2+ (x = 0.7) could significantly enhance the catalytic activity of Fe-substituted La-hexaaluminates and promote the resistance to deactivation after calcination at 1300 °C. The beneficial effect of Mg2+ is attributed to the fact that the presence of Mg2+ not only increased the distribution of Fe3+ in the mirror planes of La-hexaaluminates but also effectively suppressed the generation of catalytically inactive Fe2+. Besides, the LaMg0.7FeAl10.3O19 catalyst exhibits excellent stability for 100 h at 700 °C under the reaction conditions.

 Please wait while we load your content...
Please wait while we load your content...