Direct fabrication of catalytically active FexC sites by sol–gel autocombustion for preparing Fischer–Tropsch synthesis catalysts without reduction
Abstract
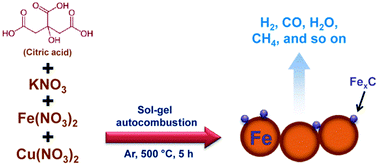
Fe-based Fischer–Tropsch synthesis (FTS) catalysts, promoted by copper and potassium, were directly prepared through a novel modified sol–gel autocombustion without further reduction. It was disclosed that the citric acid contents controlled the reduction/carburization of the catalyst, and the performance of FTS reaction was investigated. The molar ratio of citric acid to nitrates (denoted as CA/N) played a noteworthy role in the phase change of the Fe active sites and catalytic performances of the catalysts. Adding CA not only considerably improved the Fe reduction/carburization during the preparation but also the FTS catalytic performance even without a reduction process. Enhancement of the CA/N molar ratio resulted in the increase of the reducibility of catalysts. However, the FTS performance increased first and then decreased because an unnecessary reductant could lead to accumulation of the carbonic residual on the catalyst surface and decreases the catalyst performance for FTS. The FeCuK catalysts prepared using the sol–gel autocombustion method with CA as a reductant could achieve a high FTS performance without further reduction; therefore, this method could be widely applied in designing other metallic nanoparticle catalysts.

 Please wait while we load your content...
Please wait while we load your content...