Effects of calcination and activation conditions on ordered mesoporous carbon supported iron catalysts for production of lower olefins from synthesis gas†
Abstract
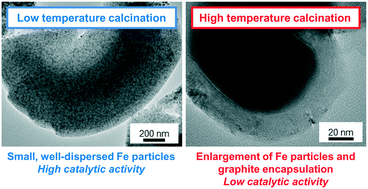
Lower C2–C4 olefins are important commodity chemicals usually produced by steam cracking of naphtha or fluid catalytic cracking of vacuum gas oil. The Fischer–Tropsch synthesis of lower olefins (FTO) with iron-based catalysts uses synthesis gas as an alternative feedstock. Nanostructured carbon materials are widely applied as supports for the iron nanoparticles due to their weak interaction with the metal species, facilitating the formation of catalytically active iron carbide. Numerous synthetic approaches towards carbon-supported FTO catalysts with various structures and properties have been published in recent years but structure-performance relationships remain poorly understood. We apply ordered mesoporous carbon (CMK-3) as a support material with well-defined pore structure to investigate the relationships between calcination/activation conditions and catalytic properties. After loading of iron and sodium/sulfur as the promoters, the structures and properties of the FTO catalysts are varied by using different calcination (300–1000 °C) and activation (350 or 450 °C) temperatures followed by FTO testing at 1 bar, 350 °C, H2/CO = 1. Carbothermal reduction of iron oxides by the support material occurs at calcination temperatures of 800 or 1000 °C, leading to a higher ratio of catalytically active iron(carbide) species but the catalytic activity remains low due to particle growth and blocking of the catalytically active sites with dense graphite layers. For the samples calcined at 300 and 500 °C, the formation of non-blocked iron carbide can be enhanced by activation at higher temperatures, leading to higher catalytic activity. Olefin selectivities of ∼60%C in the formed hydrocarbons with methane of ∼10%C are achieved for all catalysts under FTO conditions at low CO conversion. The influence of the calcination temperature is further investigated under industrially relevant FTO conditions. Promoted CMK-3-supported catalysts obtained at low calcination temperatures of 300–500 °C show stable operation for 140 h of time on stream at 10 bar, 340 °C, H2/CO = 2.


 Please wait while we load your content...
Please wait while we load your content...