2D-HfS2 as an efficient photocatalyst for water splitting
Abstract
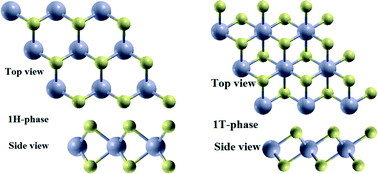
Two dimensional monolayer nanostructures for water splitting solar photocatalysts are drawing more attention due to their extraordinary properties. Using first principles calculations we have systematically investigated the structural, electronic and vibrational properties of corresponding HfS2 monolayers in both hexagonal (1H) and trigonal (1T) phases. The most stable adsorption configurations and adsorption energies are calculated. The adsorption energy of H2O on the substrate is 646.53 kJ mol−1 for the 1H-phase and 621.65 kJ mol−1 for the 1T-phase of HfS2. This shows that H2O molecules have a stronger interaction with the HfS2 substrate. The calculated redox potentials of H2O splitting lie properly astride the valence and conduction bands, suggesting that the monolayers of 1H- and 1T-HfS2 show the same characteristics as a photocatalyst for water splitting. Furthermore, we also calculated that the optical band gaps for the 1H and 1T phases of HfS2 are 2.60 eV and 3.10 eV, respectively. We have also calculated Raman spectrum signatures of the monolayer 1H and 1T-phase of the in-plane vibrational mode of the Hf and S atoms (E1g) and the out-of-plane vibrational mode of S atoms (A1g and A2u). Our work suggests that a lot more research and attention in this field is needed for the practical application of the material as visible light active photocatalysts.

 Please wait while we load your content...
Please wait while we load your content...