Alkaline versatile peroxidase by directed evolution†
Abstract
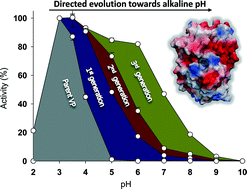
Ligninolytic peroxidases are involved in natural wood decay in strict acid environments. Despite their biotechnological interest, these high-redox potential enzymes are not functional at basic pH due to the loss of calcium ions that affects their structural integrity. In this study, we have built catalytic activity at basic pH in a versatile peroxidase (VP) previously engineered for thermostability. By using laboratory evolution and hybrid approaches, we designed an active and highly stable alkaline VP while the catalytic bases behind the alkaline activation were unveiled. A stabilizing mutational backbone allowed the pentacoordinated heme state to be maintained, and the new alkaline mutations hyperactivated the enzyme after incubation at basic pHs. The final mutant oxidises substrates at alkaline pHs both at the heme channel and at the Mn2+ site, while the catalytic tryptophan was not operational under these conditions. Mutations identified in this work could be transferred to other ligninolytic peroxidases for alkaline activation.

 Please wait while we load your content...
Please wait while we load your content...