Partial fusion of a cytochrome P450 system by carboxy-terminal attachment of putidaredoxin reductase to P450cam (CYP101A1)†
Abstract
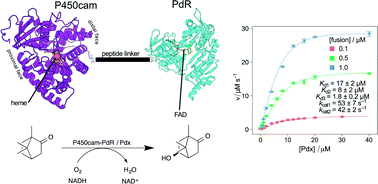
Cytochrome P450 (CYP) enzymes catalyze the insertion of oxygen into carbon–hydrogen bonds and have great potential for enzymatic synthesis. Application development of class I CYPs is hampered by their dependence on two redox partners (a ferredoxin and ferredoxin reductase), slowing catalysis compared to self-sufficient CYPs such as CYP102A1 (P450BM3). Previous attempts to address this have fused all three components in several permutations and geometries, with much reduced activity compared to the native system. We report here the new approach of fusing putidaredoxin reductase (PdR) to the carboxy-terminus of CYP101A1 (P450cam) via a linker peptide and reconstituting camphor hydroxylase activity with free putidaredoxin (Pdx). Initial purification of a P450cam–PdR fusion yielded 2.0% heme incorporation. Co-expression of E. coli ferrochelatase, lengthening the linker from 5 to 20 residues, and altering culture conditions for enzyme production furnished 85% heme content. Fusion co-expression with Pdx gave a functional system with comparable in vivo camphor oxidation activity as the native system. In vitro, the fused system's steady state NADH oxidation rate was two-fold faster than that of the native system. In contrast to the native system, NADH oxidation rates for the fusion enzyme showed non-hyperbolic dependence on Pdx concentration, suggesting a role for the PdR domain; these data were consistent with a kinetic model based on two-site binding of Pdx by P450cam–PdR and inactive dimer formation of the fusion. P450cam–PdR is the first example of a class I P450 fusion that exhibits significantly more favorable behavior than that of the native system.


 Please wait while we load your content...
Please wait while we load your content...