Siliceous tin phosphates as effective bifunctional catalysts for selective conversion of dihydroxyacetone to lactic acid†
Abstract
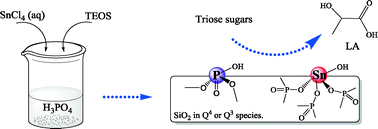
Methods to catalytically convert carbohydrates into lactic acid (LA), which is a versatile platform chemical, have been widely investigated. In this study, siliceous tin phosphates were utilized as reusable Brønsted–Lewis acid bifunctional catalysts during the conversion of 1,3-dihydroxyacetone (DHA) to LA under hydrothermal conditions. The product distribution closely depended on the reaction temperature, catalyst loading and substrate concentration. The highest LA yield of 93.8% was achieved with a complete DHA conversion at 140 °C after 5 h. The reaction was facilitated by the vast presence of Brønsted and Lewis acid sites that were confirmed by both pyridine FTIR and NH3-TPD analysis. The incorporation of silica significantly lowered the Sn content and improved the thermal stability of the tin phosphate catalysts. A possible reaction mechanism was proposed in that the Lewis and Brønsted acid sites synergistically catalyzed the conversion of pyruvaldehyde to LA, which was found to be the rate-determining step. The method allows for facile catalyst separation and recycling while expanding the applicability of silica in the field of biomass-to-chemical conversion.

 Please wait while we load your content...
Please wait while we load your content...