Hydrogen bond-directed encapsulation of metalloporphyrin into the microcages of zeolite imidazolate frameworks for synergistic biomimetic catalysis†‡
Abstract
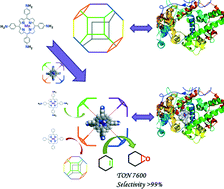
In efforts to replicate the 3D model and desirable function of haemoglobin, the zeolite imidazolate framework (ZIF-8) was delineated for an ideal host matrix to accommodate custom-designed porphyrin molecules via hydrogen bonding, thus providing a suitable micro-environment similar to the protein skeleton around the active sites in haemoglobin. The spectral investigations including X-ray photoelectron spectroscopy (XPS) tests and Raman spectra, together with the experimental details and computational simulations, showed that it is a weak hydrogen bond that drives guests into the cage of ZIF-8. At the atomic level, the amino substituent on Mn-TAPP coupled with the C–H on the 2-methylimidazolate (MeIM) constitutes the host–guest interaction (Por-N⋯H-MeIM). In particular, this novel heterogeneous catalyst, denoted as Mn-TAPP@ZIF-8, shows an enhanced catalytic efficiency (up to 7600 ton) compared to the homogeneous complexes and other reported studies, strongly supporting the synergistic interplay raised from the hydrogen bond.

 Please wait while we load your content...
Please wait while we load your content...