Esterification of fatty acids from waste cooking oil to biodiesel over a sulfonated resin/PVA composite†
Abstract
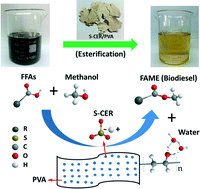
Sulfonated cation exchange resins (s-CERs) have been widely studied as a replacement of liquid acids for the catalysis of esterification of free fatty acids (FFAs) to produce biodiesel with water as the only by-product. However, the water produced has strong affinity to sulfonate groups in s-CERs, which block the reactive sites for esterification and thus reduce the activity of a catalyst. To overcome this technical barrier, we have designed an s-CER/PVA composite by incorporating s-CER fines within a poly(vinyl alcohol) (PVA) matrix. PVA has a much stronger absorption preference for water than s-CERs and has very low selectivity for reactants (FFAs and methanol), which enables continuous removal of the produced water and liberation of reactive sulfonate sites in s-CERs for catalysis. With s-CER/PVA, FFA conversion was increased from 80.1% to 97.5% after an 8-hour reaction and the turnover frequency (TOF) was increased more than 3.3 times. The TOF of s-CER/PVA was also 2.6 times higher than that of sulfuric acid, suggesting that water-less, heterogeneous sulfonate sites are more reactive than water-blocked homogeneous ones. The reusability of s-CER/PVA was also enhanced due to the fact that the produced water that could cause deactivation of the s-CERs was largely removed by PVA.


 Please wait while we load your content...
Please wait while we load your content...