Semiempirical QM/MM calculations reveal a step-wise proton transfer and an unusual thiolate pocket in the mechanism of the unique arylpropionate racemase AMDase G74C†
Abstract
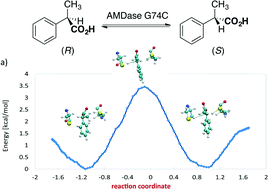
The mechanism of the unique arylpropionate racemase AMDase G74C was investigated by a QM/MM approach. Molecular dynamics simulations showed that the mechanism is initiated by a deprotonation of the catalytic cysteine. The simulations revealed two thiolate pockets. While the first plays a role in the natural decarboxylative activity of AMDase, the second stabilizes the artificially introduced thiolate group of C74. The presence of the two structural motifs is a prerequisite for the promiscuous racemization reaction of AMDase G74C. QM/MM simulations show that the deprotonation and reprotonation proceed in a stepwise fashion, in which a planar enedionate intermediate is stabilized by a delocalized π-electron system on a vinylic or aromatic substituent of the substrate. The artificial racemase is thus a typical case of substrate-assisted catalysis.

 Please wait while we load your content...
Please wait while we load your content...