Defect stabilized gold atoms on graphene as potential catalysts for ethylene epoxidation: a first-principles investigation†‡
Abstract
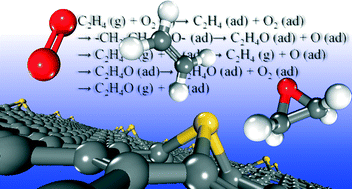
We performed a first-principles based investigation on the potential role of Au atoms stabilized by defects on graphene in ethylene epoxidation. We showed that the interactions between the Au atoms and vacancies on graphene not only make the Au atomic diffusion a 2.10 eV endothermic process, but also tune the energy level of Au-d states for the activation of O2 and ethylene and promote the formation and dissociation of the peroxametallacycle intermediate. The catalytic cycle of ethylene epoxidation is initiated with the formation of a peroxametallacycle intermediate by the coadsorbed ethylene and O2, through the dissociation of which an ethylene epoxide molecule and an adsorbed O atom are formed. Then, gaseous ethylene reacts with the remnant O atom directly for the formation of another ethylene epoxide molecule. The desorption of ethylene epoxide is facilitated by the subsequent adsorption of O2 or ethylene and a new reaction cycle initiates. The calculated energy barriers for the formation and dissociation of the peroxametallacycle intermediate and the regeneration of Au sites are 0.30, 0.84 and 0.18 eV, respectively, and are significantly lower than those for aldehyde formation. These findings suggest the potential high catalytic performance of these Au atoms for ethylene epoxidation.

 Please wait while we load your content...
Please wait while we load your content...