Room-temperature catalytic oxidation of alcohols with the polyoxovanadate salt Cs5(V14As8O42Cl)†
Abstract
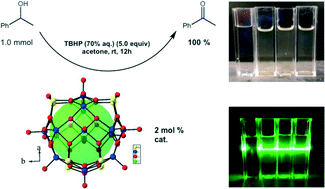
While many known methods for oxidation mediated by polyoxometalates (POMs) employ environmentally friendly co-oxidants, they tend to employ large catalyst loadings (e.g. 40 mol%) and costly high reaction temperatures (~90–135 °C) that potentially contribute to the degradation of the catalyst and reduce their effectiveness. Herein, we present some initial results demonstrating a room temperature catalytic oxidation using the reduced salt-inclusion polyoxometalate, Cs5(V14As8O42Cl), that contains polyoxovanadate (POV) clusters as an efficient catalyst (e.g., 2 mol%) in the transformation of secondary alcohols to their corresponding ketones in very good to quantitative yields. Further, the catalyst can be suspended on celite and recycled.

 Please wait while we load your content...
Please wait while we load your content...