Microporous crystalline Mo–V mixed oxides for selective oxidations
Abstract
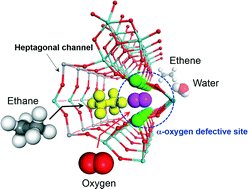
Recent developments of crystalline Mo3VOx catalysts (MoVO), a new type of oxidation catalysts for selective oxidations of ethane to ethene and of acrolein to acrylic acid, are reviewed. MoVO are formed by the building unit assembly of polyoxomolybdates under hydrothermal conditions. These catalysts are composed of a network arrangement based on a {Mo6O21}6− pentagonal unit and a {MO6} (M = Mo, V) octahedral unit to form a hexagonal channel and a heptagonal channel. Between these channels, the heptagonal channel acts as a micropore of 0.40 nm in diameter which can adsorb small molecules such as CO2, N2, methane, ethane, etc. The size of the heptagonal channel micropore is reversibly and continuously tunable by redox treatment. Interestingly, the heptagonal channel activates ethane inside and acrolein on the channel located over the external surface. Tuning of the heptagonal channel size significantly modifies the catalytic performance for the selective oxidation of ethane. Strong relationships among crystal structure, microporosity, and catalytic performance were observed here.

 Please wait while we load your content...
Please wait while we load your content...