Rational design of ethanol steam reforming catalyst based on analysis of Ni/La2O3 metal–support interactions†
Abstract
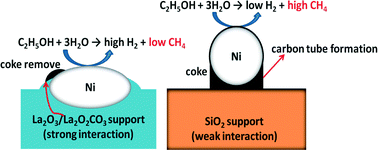
Understanding the metal–support interactions between Ni ansd La2O3 has helped us design an improved catalyst for the ethanol steam reforming (ESR) reaction. Information from in situ X-ray absorption spectra (XAS) and high-resolution transmission electron microscopy (HRTEM) has helped us to prepare a Ni-based catalyst, in which strong metal–support interactions (SMSI) maximize the hydrogen yield by suppressing undesired reaction pathways. It was found that the Ni, formed as nanoparticles, was well dispersed both in and on the La2O3. The Ni/La2O3 catalyst, when compared to a Ni/SiO2 catalyst, yielded twice as much H2 (3.7 mol H2 per mol EtOH) at 395 °C by both inhibiting methanation (CO + 3H2 → CH4 + H2O) and promoting the water gas shift reaction (CO + H2O → CO2 + H2). It is expected that our enhanced understanding of Ni/La2O3 physical/chemical interactions will help us design new catalysts for various catalytic applications.


 Please wait while we load your content...
Please wait while we load your content...