Old tricks, new dogs: organocatalytic dienamine activation of α,β-unsaturated aldehydes
Abstract
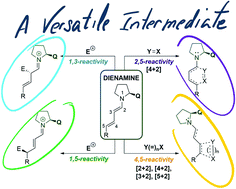
Chiral secondary amines are some of the most commonly used kinds of catalysts. They have become a reliable tool for the α- and β-activation of carbonyl compounds, via HOMO, SOMO or LUMO activation pathways. Recently, chemists have turned their attention to the development of novel organocatalytic strategies for remote functionalisation, targeting stereocentres even more distant from the catalyst-activation site, through dienamine, trienamine, and vinylogous iminium ion pathways (γ-, ε- and δ-positions, respectively). Here we outline and discuss the state-of-the-art in dienamine activation, classifying examples according to the different reactive activation pathways followed by the formed dienamine intermediate (1,3-, 1,5-, 2,5- and 4,5-functionalisation) and the reaction type developed, as determined by the structure and the nature of electrophiles and nucleophiles.


 Please wait while we load your content...
Please wait while we load your content...