Photoinduced charge separation in wide-band capturing, multi-modular bis(donor styryl)BODIPY–fullerene systems†
Abstract
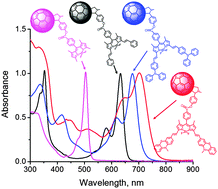
A new series of multi-modular donor–acceptor systems capable of exhibiting photoinduced charge separation have been designed, synthesized and characterized using various techniques. In this series, the electron donor was a BF2-chelated dipyrromethene (BODIPY) appended with two styryl linkers carrying two electron rich triphenylamine or phenothiazine entities. Fulleropyrrolidine linked at the meso-position of the BODIPY ring served as an electron acceptor. As a result of extended conjugation and multiple electroactive chromophore entities, the bis-styryl BODIPY revealed absorbance and emission well-into the near-infrared region covering a 300–850 nm spectral range. Using redox, computational, absorbance and emission data, an energy level diagram was constructed that helped in envisioning the different photochemical events. Spectral evidence for photoinduced charge separation in these systems was established from femtosecond and nanosecond transient absorption studies. The measured rate constants indicated fast charge separation and relatively slow charge recombination revealing their usefulness in light energy harvesting and optoelectronic device building applications. The bis(donor styryl)BODIPY–fullerene systems populated BODIPY triplet excited states during the process of charge recombination.

 Please wait while we load your content...
Please wait while we load your content...