Ammonia as an efficient catalyst for decomposition of carbonic acid: a quantum chemical investigation†
Abstract
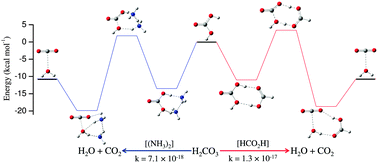
Electronic structure calculations using M06-2X, MP2 and CCSD(T) methods have been employed to show ammonia as an efficient catalyst for decomposition of carbonic acid. The results predict that ammonia can catalyze the reaction as both a monomer and dimer, the latter being more efficient as it makes the reaction nearly barrierless. It has been shown that monomeric ammonia makes the process significantly faster compared with the water monomer (the rate constant being 104 to 105 times higher) as well as the water dimer (10–20 times faster). Dimeric ammonia has been shown to be a better catalyst than its monomeric counterpart (the rate constant being 103 to 104 times higher). Its efficiency as a catalyst was found to be close to that of formic acid. Owing to the fact that ammonia is present in the Earth's atmosphere at a significant trace level, it is expected to play a nontrivial, if not pivotal, role in atmospheric chemistry as a catalyst.

 Please wait while we load your content...
Please wait while we load your content...