Positive and negative linear compressibility and electronic properties of energetic and porous hybrid crystals with nitrate anions
Abstract
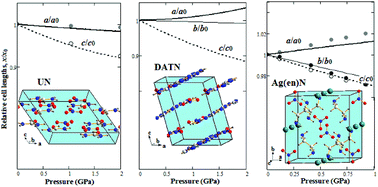
The structural and electronic properties of energetic nitrates with organic cations (uronium and 3,3′-diamino-4,4′-azo-1,2,4-triazole) and a metal–organic framework crystal [Ag(ethylenediamine)]NO3 have been investigated using density functional theory including van der Waals interactions. It is found that the linear compressibility of urea nitrate is positive and anisotropic (a ≈ b < c), whereas 3,3′-diamino-4,4′-azo-1,2,4-triazole nitrate and [Ag(ethylenediamine)]NO3 show both positive and negative linear compressibility along the b, c and a-axes, respectively. Negative linear compressibility is correlated with the expansion of hydrogen bonds. The band gaps of considered crystals are different, which is related to the difference in the nature (anionic, cationic or mixed) of upper valence and lower unoccupied electronic states. The band gap of 3,3′-diamino-4,4′-azo-1,2,4-triazole nitrate is the smallest and nonlinearly decreases with pressure.

 Please wait while we load your content...
Please wait while we load your content...