125Te NMR provides evidence of autoassociation of organo-ditellurides in solution†
Abstract
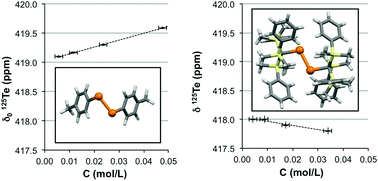
The frequency of the resonance of 125Te of two organo-ditellurides, R–Te–Te–R (R = 4-CH3C6H4 and 2-(CH3)2NCH2C6H4), in solution undergoes a low-field shift as the concentration of the sample increases. In sharp contrast, the resonance of a sterically hindered ditelluride (R = (C6H5(CH3)2Si)3C) and telluric acid display the opposite effect. While the negative concentration coefficients can be explained by the change in magnetic susceptibility, the positive coefficients are consistent with autoassociation of the molecules through tellurium-centred supramolecular interactions. Although the corresponding equilibrium constants are small, the process is shown to be exothermic. However, the influence of autoassociation is much smaller than the effects of solvent polarity and the conformation of the ditelluride bond.


 Please wait while we load your content...
Please wait while we load your content...