Electrons, excitons and hydrogen bonding: electron-promoted desorption from molecular ice surfaces†
Abstract
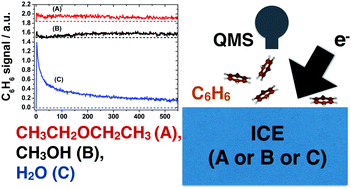
Desorption of benzene (C6H6) from thick methanol (CH3OH) and diethyl ether (CH3CH2OCH2CH3) ices during irradiation with 250 eV electrons is reported and compared with our previous work on C6H6 desorption from water (H2O) ice systems. C6H6 electron-promoted desorption (EPD) is seen to be sensitive to the chemical nature of the substrate reflecting both the importance of the excitations localised around the O-atom versus those involving the C-atom; and the role of hydrogen bonding interactions in transporting non-dissociative electronic excitation to the substrate/C6H6 interfaces during the electron irradiation.

 Please wait while we load your content...
Please wait while we load your content...