Insights into the effect of CO2 absorption on the ionic mobility of ionic liquids†
Abstract
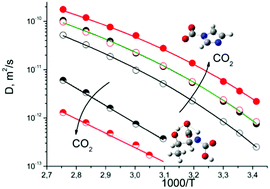
We investigate a comparative effect of CO2 absorption on the ionic mobility of two choline based ionic liquids comprising two different anions such as threonine and imidazole. The synthesized ionic liquids were characterized using 1H and 13C NMR and other spectroscopic techniques. By keeping a common cation and changing the anion from threonine to imidazole both the viscosity and density reduced drastically. We found that [N1,1,6,2OH][Imi] exhibits the highest CO2 capture capacity at 20 °C of 5.27 mol of CO2 per kg of ionic liquid (1.27 mol of CO2 per mol of ionic liquid, 23.26 wt% of CO2) whereas [N1,1,6,2OH][Threo] exhibits 3.6 mol of CO2 per kg of ionic liquid (1.05 mol of CO2 per mol of ionic liquid, 15.87 wt% of CO2). The activation energy for diffusion is calculated using the Vogel-Fulcher-Tamman (VFT) equation in the form of diffusivity. It was found that the activation energy for the diffusion of [N1,1,6,2OH][Threo] is ∼10 times higher than that of [N1,1,6,2OH][Imi]. 1H diffusion NMR data revealed that the diffusivity of [N1,1,6,2OH][Imi] is increased after CO2 absorption whereas a decrease in diffusivity was observed in the case of [N1,1,6,2OH][Threo]. This anomalous behavior of [N1,1,6,2OH][Imi] was further explained by using DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...