Revisiting the F + HCl → HF + Cl reaction using a multireference coupled-cluster method†
Abstract
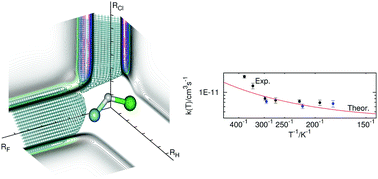
We have constructed a new potential energy surface for the title reaction, based on the internally contracted multireference coupled-cluster method. The calculated barrier height is 1.59 ± 0.08 kcal mol−1. This value is much lower than that obtained in previous ab initio calculations and it is close to the experimentally suggested value. Other features of the [F,H,Cl] system are also analysed, such as van der Waals minima and conical intersections. The rate constant and the vibrational and rotational distributions of the products were calculated using a fully converged time independent quantum mechanical approach. The calculated rate constant agrees well with the experimental values. Qualitative agreement for the vibrational distribution is obtained and it is shown that it is strongly influenced by the initial rotational state distribution.

 Please wait while we load your content...
Please wait while we load your content...