Neurotrophin-mimicking peptides at the biointerface with gold respond to copper ion stimuli
Abstract
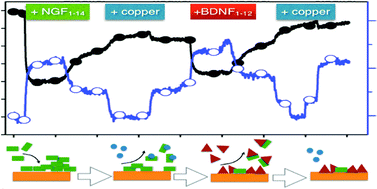
The peptide fragments NGF1–14 and BDNF1–12, encompassing the N-terminal domains, respectively, of the proteins nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) were used in this study for the fabrication of a hybrid gold/peptide biointerface. These peptides mimic the Trk receptor activation of the respective whole protein – with a crucial role played by copper ions – and exhibit, in bulk solution, a pH-dependent capability to complex copper. We demonstrate here the maintenance of peptide-specific responses at different pH values as well as the copper binding also for the adlayers formed upon physisorption at the gold surface. The physicochemical properties, including viscoelastic behavior of the adlayer and competitive vs. synergic interactions in sequential adsorption processes, were addressed both experimentally, by quartz crystal microbalance with dissipation monitoring (QCM-D) and circular dichroism (CD), and theoretically, by molecular dynamics (MD) calculations. Proof-of work biological assays with the neuroblastoma SY-SH5H cell line demonstrated that the developed hybrid Au/peptide nanoplatforms are very promising for implementation in pH- and metal-responsive systems for application in nanomedicine.


 Please wait while we load your content...
Please wait while we load your content...