Strong 1D localization and highly anisotropic electron–hole masses in heavy-halogen functionalized graphenes†
Abstract
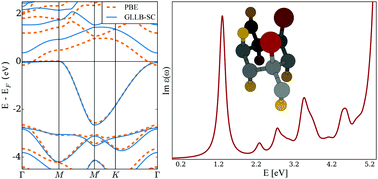
While halogenation of graphene presents a fascinating avenue to the construction of a chemically and physically diverse class of systems, their application in photovoltaics has been hindered by often prohibitively large optical gaps. Herein we study the effects of partial bromination and chlorination on the structure and optoelectronic properties of both graphane and fluorographene. We find brominated and chlorinated fluorographene derivatives to be as stable as graphane with a detailed investigation of the systems band structure revealing significant 1D localization of the charge carriers as well as strongly electron–hole asymmetric effective masses. Lastly using G0W0 and BSE, we investigate the optical adsorption spectra of the aforementioned materials whose first adsorption peak is shown to lie close to the optimal peak position for photovoltaic applications (≈1.5 eV).

 Please wait while we load your content...
Please wait while we load your content...