Ab initio molecular dynamics study of Se(iv) species in aqueous environment
Abstract
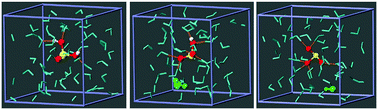
An ab initio molecular dynamics investigation is carried out on various water-borne Se(IV) species, H2SeO3, HSeO3− and SeO32−, in aqueous environment. Consistent with the reported acid dissociation constants, in neutral solution H2SeO3 exchanges protons with the surrounding water molecules establishing a dynamic equilibrium with HSeO3−. The SeO32− species is found to be stable only in basic environment, which is emulated in the present simulation through introducing a hydroxide ion, OH−, in the system. The hydration structure, hydrogen bonding and spectroscopic signatures of the species are comprehensively analyzed. The influence of the solute's hydration structure on the structural and dynamic response of the solvent is discussed. The correlation between the strength as well as the number of hydrogen bonds accepted by the solute on its vibrational properties are analyzed.

 Please wait while we load your content...
Please wait while we load your content...