Single oxygen vacancies of (TiO2)35 as a prototype reduced nanoparticle: implication for photocatalytic activity†
Abstract
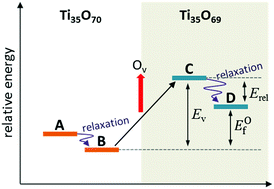
Titanium dioxide (TiO2), as a semiconductor metal oxide, has been one of the most popular materials studied in the field of photocatalysis. In the present study, the properties of single oxygen vacancies of (TiO2)35, a prototype of an anatase nanoparticle, were investigated by DFT calculations. (TiO2)35 is the minimum sized model (∼2 nm) for a bipyramidal nanoparticle with anatase phase and eight {101} facets. All the available oxygen vacancies at various sites according to position, coordination number, and distance from the center atom were examined. The geometric, energetic and electronic properties of the reduced TiO2 clusters were analyzed by hybrid DFT functionals with different Hartree–Fock exchange ratios (0, 12.5 and 25%). It was found that the structure of pristine (TiO2)35 is somewhat different from the bulk lattice, with a relatively high surface to volume ratio. Moreover, the particular highly (three)-coordinated oxygen atom is energetically the most favorable for oxygen vacancy formation from the nanoparticle mainly due to its substantially high relaxation energy. TiO2 nanoparticles have low oxygen vacancy formation energy and narrow band gap because of their defect states, and can be utilized as an efficient photocatalyst material.

 Please wait while we load your content...
Please wait while we load your content...