Concentric dual π aromaticity in bowl-like B30 cluster: an all-boron analogue of corannulene†
Abstract
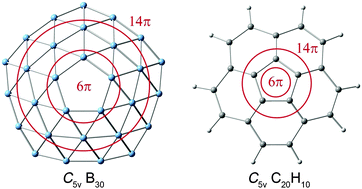
A chemical bonding model is presented for the bowl-like C5v B30 global-minimum cluster with a central pentagonal hole. The B30 cluster is composed of three concentric boron rings: first B5, second B10, and third B15. The first and second B rings constitute an inner double-chain ribbon and support a delocalized π sextet. The second and third rings form an outer double-chain ribbon, where 14π delocalized electrons are situated. The unique π systems lead to concentric dual π aromaticity for B30, a concept established from concerted computational data on the bases of canonical molecular orbital (CMO) analysis, adaptive natural density partitioning (AdNDP), nucleus-independent chemical shifts (NICS), and natural charge calculations. A proposal is put forward that the bowl-like B30 cluster is an exact all-boron analogue of corannulene (C20H10), a fragment of C60 fullerene. The bonding nature of corannulene is revisited and fully elucidated herein. A comparison of the bonding patterns in bowl-like C5v B30 cluster and two other structural isomers (Cs and C1) unravels the mechanism as to why the defective hole prefers to be positioned at the center.

 Please wait while we load your content...
Please wait while we load your content...