Effects of water molecules on the chemical stability of MAGeI3 perovskite explored from a theoretical viewpoint†
Abstract
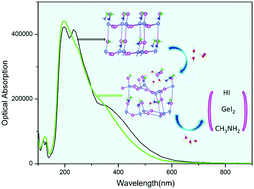
The stability of perovskite in humid environments is one of the biggest obstacles for its potential applications in light harvesting and electroluminescent displays. Understanding the detailed degradation mechanism of MAGeI3 in moisture is a critical way to explore the practicability of MAGeI3 perovskite. In this study, we report a quantitative and systematic investigation of MAGeI3 degradation processes by exploring the effects of H2O molecules on the structural and electronic properties of the most stable MAGeI3(101) surface under various simulated environmental conditions with different water coverage based on first-principles calculations. The results show that H2O molecules can easily diffuse into the inner side of the perovskite and gradually corrode the structure as the number of H2O molecules increases. As a result of the interactions between perovskite and H2O molecules, a hydrated intermediate will be generated as the first step in the degradation mechanism; the perovskite will further decompose to HI and GeI2. In terms of one MAGeI3 molecule, it will be dissociated completely to GeI2 as a result of hydrolysis reactions with a minimum of 4H2O molecules. In addition, the degradation of the perovskite will also affect the electronic structure, causing a decrease in optical absorption across the visible region of the spectrum and a distinct deformation change in the crystal structure of the material. These findings further illustrate the degradation of the hydrolysis process of MAGeI3 perovskite in humid environments, which should be helpful to inspire experimentalists to take action to prolong the lifetimes of perovskite solar cells to achieve high conversion efficiency in their applications.

 Please wait while we load your content...
Please wait while we load your content...