The influence of mass-transport conditions on the ethanol oxidation reaction (EOR) mechanism of Pt/C electrocatalysts
Abstract
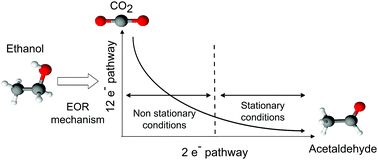
This study aims to provide further understanding of the influence of different parameters that control mass-transport (the revolution rate of the rotating disk electrode and the potential scan rate) on the ethanol oxidation reaction (EOR). The experiments were conducted on a home-made carbon-supported 20 wt% Pt/C electrocatalyst, synthesized using a modified polyol method, and characterized in terms of physicochemical properties by thermogravimetric analysis (TGA), powder X-ray diffraction (XRD) and transmission electron microscopy (TEM). The EOR at the thin active layer of this electrocatalyst was characterized using both differential electrochemical mass spectrometry (DEMS) in a flow cell configuration and the rotating disc electrode (RDE). The results demonstrate that operating under stationary conditions (low scan rate and high RDE speed) hinders complete ethanol electrooxidation into CO2 and favors the poisoning of the electrocatalyst surface by hydroxide and strong ethanol adsorbates. As such, the EOR appears to be more efficient and faster under dynamic conditions than in near steady-state.

 Please wait while we load your content...
Please wait while we load your content...