Comparison of biological chromophores: photophysical properties of cyanophenylalanine derivatives†
Abstract
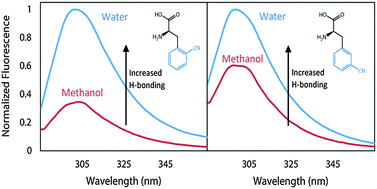
Within this work, the family of cyanophenylalanine spectroscopic reporters is extended by showing the ortho and meta derivatives have intrinsic photophysical properties that are useful for studies of protein structure and dynamics. The molar absorptivities of 2-cyanophenylalanine and 3-cyanophenylalanine are shown to be comparable to that of 4-cyanophenylalanine with similar spectral features in their absorbance and emission profiles, demonstrating that these probes can be utilized interchangeably. The fluorescence quantum yields are also on the same scale as commonly used fluorophores in peptides and proteins, tyrosine and tryptophan. These new cyano-fluorophores can be paired with either 4-cyanophenylalanine or tryptophan to capture distances in peptide structure through Förster resonance energy transfer. Additionally, the spectroscopic properties of these chromophores can report the local solvent environment via changes in fluorescence emission intensity as a result of hydrogen bonding and/or hydration. A decrease in the quantum yield is also observed in basic environments due to photoinduced electron transfer from a deprotonated amine in the free PheCN species and at the N-terminus of a short peptide, providing an avenue to detect pH in biological systems. Our results show the potential of these probes, 2-cyanophenylalanine and 3-cyanophenylalanine, to be incorporated into a single peptide chain, either individually or in tandem with 4-cyanophenylalanine, tryptophan, or tyrosine, in order to obtain information about peptide structure and dynamics.

 Please wait while we load your content...
Please wait while we load your content...