Aqueous biphasic systems composed of ionic liquids and polypropylene glycol: insights into their liquid–liquid demixing mechanisms†
Abstract
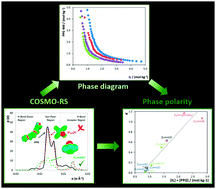
Novel ternary phase diagrams of aqueous biphasic systems (ABSs) composed of polypropylene glycol with an average molecular weight of 400 g mol−1 (PPG-400) and a vast number of ionic liquids (ILs) were determined. The large array of selected ILs allowed us to evaluate their tuneable structural features, namely the effect of the anion nature, cation core and cation alkyl side chain length on the phase behaviour. Additional evidence on the molecular-level mechanisms which rule the phase splitting was obtained by 1H NMR (Nuclear Magnetic Resonance) spectroscopy and by COSMO-RS (Conductor-like Screening Model for Real Solvents). Some systems, for which the IL–PPG-400 pairs are completely miscible, revealed to be of type “0”. All data collected suggest that the formation of PPG–IL-based ABSs is controlled by the interactions established between the IL and PPG, contrarily to previous reports where a “salting-out” phenomenon exerted by the IL over the polymer in aqueous media was proposed as the dominant effect in ABS formation. The influence of temperature on the liquid–liquid demixing was also evaluated. In general, an increase in temperature favours the formation of an ABS in agreement with the lower critical solution temperature (LCST) phase behaviour usually observed in polymer–IL binary mixtures. Partition results of a dye (chloroanilic acid, in its neutral form) further confirm the possibility of tailoring the phases' polarities of IL–PPG-based ABSs.


 Please wait while we load your content...
Please wait while we load your content...