Abstract
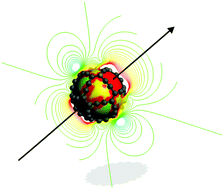
A spherical and cavernous carbocage molecule exhibiting faces with larger ring sizes than regular fullerenes is a suitable species for investigating how molecular magnetic properties depend on the structure of the molecular framework. The studied all-carbon gaudiene (C72) is a highly symmetrical molecule with three- and four-fold faces formed by twelve membered rings. Here, we attempt to unravel the magnetic response properties of C72 by performing magnetic shielding and current density calculations with the external magnetic field applied in different directions. The obtained results indicate that the induced current density flows mainly along the chemical bonds that are largely perpendicular to the magnetic field direction. However, the overall current strength for different directions of the magnetic field is nearly isotropic differing by only 10% indicating that C72 can to some extent be considered to be a spherical aromatic molecule, whose current density and magnetic shielding are ideally completely isotropic. The induced magnetic field is found to exhibit long-range shielding cones in the field direction with a small deshielding region located perpendicularly to the field outside the molecule. The magnetic shielding is isotropic inside the molecular framework of C72, whereas an orientation-dependent magnetic response appears mainly at the exterior of the molecular cage.


 Please wait while we load your content...
Please wait while we load your content...