Under what conditions does (SiO)N nucleation occur? A bottom-up kinetic modelling evaluation†
Abstract
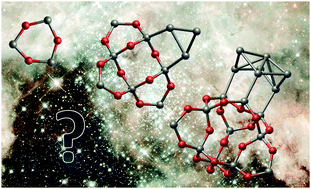
Silicon monoxide (SiO) is a structurally complex compound exhibiting differentiated oxide-rich and silicon-rich nano-phases at length scales covering nanoclusters to the bulk. Although nano-sized and nano-segregated SiO has great technological potential (e.g. nano-silicon for optical applications) and is of enormous astronomical interest (e.g. formation of silicate cosmic dust) an accurate general description of SiO nucleation is lacking. Avoiding the deficiencies of a bulk-averaged approach typified by classical nucleation theory (CNT) we employ a bottom-up kinetic model which fully takes into account the atomistic details involved in segregation. Specifically, we derive a new low energy benchmark set of segregated (SiO)N cluster ground state candidates for N ≤ 20 and use the accurately calculated properties of these isomers to calculate SiO nucleation rates. We thus provide a state-of-the art evaluation of the range of pressure and temperature conditions for which formation of SiO will or will not proceed. Our results, which match with available experiment, reveal significant deficiencies with CNT approaches. We employ our model to shed light on controversial issue of circumstellar silicate dust formation showing that, at variance with the predictions from CNT-based calculations, pure SiO nucleation under such conditions is not viable.


 Please wait while we load your content...
Please wait while we load your content...