Quasi-chemical approximation for polyatomic mixtures
Abstract
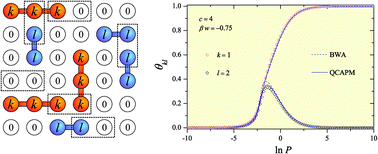
The statistical thermodynamics of binary mixtures of polyatomic species was developed based on a generalization in the spirit of the lattice-gas model and the quasi-chemical approximation (QCA). The new theoretical framework is obtained by combining: (i) the exact analytical expression for the partition function of non-interacting mixtures of linear k-mers and l-mers (species occupying k sites and l sites, respectively) adsorbed in one dimension, and its extension to higher dimensions; and (ii) a generalization of the classical QCA for multicomponent adsorbates and multisite-occupancy adsorption. This process is analyzed using the partial adsorption isotherms corresponding to both species of the mixture. Comparisons with analytical data from Bragg–Williams approximation (BWA) and Monte Carlo simulations are performed in order to test the validity of the theoretical model. Even though a good fitting is obtained from BWA, it is found that QCA provides a more accurate description of the phenomenon of adsorption of interacting polyatomic mixtures.

 Please wait while we load your content...
Please wait while we load your content...