On the effect of ion pairing of Keggin type polyanions with quaternary ammonium cations on redox potentials in organic solvents†
Abstract
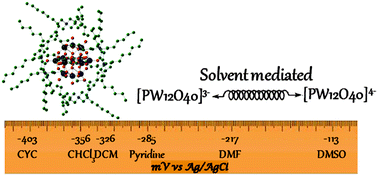
The electrochemical properties of Keggin type polyoxometalates Qn[XW12O40] (X = P, Si, B; Q = n-tetraoctylammonium and n-trioctylmethylammonium) in organic solvents were investigated in order to understand the interrelation between the redox potentials, solvents and ion pairing. A logarithmic correlation between the dielectric constant of the solvent (ε ranged from 4.8 to 46.6) and the reduction potential of the [PW12O4]3−/[PW12O4]4− couple was found. This reduction potential increased significantly when the surface charge of the polyoxometalate went from 3− to 5−. The investigation of the ion pairing properties by diffusion NMR revealed the presence of intimate ion pairs in less polar solvents (e.g. dichloromethane) and less compact ion pairs in more polar solvents (e.g. DMSO). Using a V atom within the polyoxometalate an NMR experiment showed that a n-trioctylmethyl ammonium cation bonded to the polyoxometalate anion more intimately than a n-tetraoctyl ammonium cation.

 Please wait while we load your content...
Please wait while we load your content...