A guide to the selection of switchable functional groups for CO2-switchable compounds†
Abstract
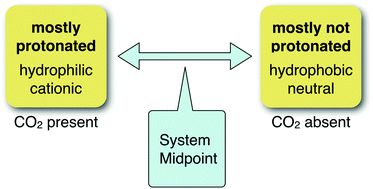
Many CO2-responsive species, including many of the CO2-switchable surfactants, solvents, solutes, gels, colloids, and surfaces, rely on the ability of CO2 to lower the pH of water. Uncharged basic groups on the CO2-responsive species are therefore converted from a neutral state to a protonated cationic state (a bicarbonate salt), which causes dramatic and useful changes to the properties of the species. However, this switching process only works correctly if a basic group of appropriate basicity has been selected. This article presents a comprehensive guide to the selection of basic groups for CO2-switchable species for use in water. The appropriate basicity, as measured by the pKaH (the pKa of the protonated compound), is a function of the concentration of the switchable species, the temperature, the pressure of CO2, the presence or absence of an organic liquid phase, and the solubility of the neutral form of the compound.

 Please wait while we load your content...
Please wait while we load your content...