Photosensitized reactions initiated by 6-carboxypterin: singlet and triplet reactivity†
Abstract
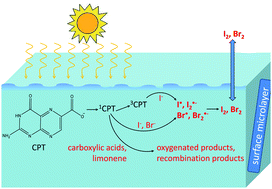
Pterins, derivatives of 2-aminopteridin-4(3H)-one, are natural photosensitizers, common to many biological systems. Indications that these photosensitizers are also present in the sea-surface microlayer motivated the study of the photophysical and photochemical properties of 6-carboxypterin (CPT), which was chosen as a model for this group of photoactive compounds. The kinetics of excited CPT in the singlet and triplet state in the presence of halides and organics were studied in aqueous solutions at neutral pH by means of steady-state fluorescence and laser-flash photolysis. The fluorescence of CPT was efficiently quenched by two halides (iodide and bromide) and by four carboxylic acids (lactic, malonic, propionic and citric acid) with reaction rates close to the diffusion-controlled limit. In the triplet state, the triplet absorption spectrum was measured and its pH dependence was studied. The triplet state of CPT showed relatively high reactivity towards iodide, but no reaction with bromide or chloride could be observed. No singlet or triplet state quenching in the presence of limonene could be measured. A reaction mechanism is proposed, initiated by electron transfer from the quencher to the excited photosensitizer. This type of photo-induced reaction in the sea-surface microlayer has the potential to trigger the production of many oxidized species, including halogen atoms, in the bulk and gaseous phases.

 Please wait while we load your content...
Please wait while we load your content...