Spectroscopic study on the active site of a SiO2 supported niobia catalyst used for the gas-phase Beckmann rearrangement of cyclohexanone oxime to ε-caprolactam
Abstract
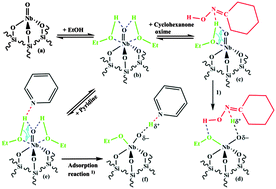
NbOx/SiO2 with a very high catalytic activity for the gas-phase Beckmann rearrangement of cyclohexanone oxime to ε-caprolactam, was investigated by different spectroscopic methods in order to obtain new insights in the formation and nature of the active sites. FT-IR spectroscopy in combination with pyridine adsorption measurements revealed that the catalyst material contains Lewis-acidic sites, most probably related to the Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of isolated tetrahedral NbO4 surface species, whereas no Brønsted-acidic sites were observed. Results from in situ Raman and complementary FT-IR measurements strongly suggest that Brønsted-acidic Nb–OH sites can be generated from Nb
O groups of isolated tetrahedral NbO4 surface species, whereas no Brønsted-acidic sites were observed. Results from in situ Raman and complementary FT-IR measurements strongly suggest that Brønsted-acidic Nb–OH sites can be generated from Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups by reaction with ethanol. This is in agreement with the observation that ethanol is essential for obtaining a very good catalyst performance. However, the Brønsted-acidic sites can be detected in significant amounts in particular in the presence of a Lewis-base, e.g. pyridine, most probably because the formation and/or the stability of these Brønsted-acidic sites are enhanced by a basic molecule. Assuming that cyclohexanone oxime, being a base, can play a similar role as pyridine, we propose on the basis of the spectroscopic findings obtained in this work and our kinetic results published recently, a reaction scheme for the formation of the active site at the Nb
O groups by reaction with ethanol. This is in agreement with the observation that ethanol is essential for obtaining a very good catalyst performance. However, the Brønsted-acidic sites can be detected in significant amounts in particular in the presence of a Lewis-base, e.g. pyridine, most probably because the formation and/or the stability of these Brønsted-acidic sites are enhanced by a basic molecule. Assuming that cyclohexanone oxime, being a base, can play a similar role as pyridine, we propose on the basis of the spectroscopic findings obtained in this work and our kinetic results published recently, a reaction scheme for the formation of the active site at the Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group as well as for the recovery of the Nb
O group as well as for the recovery of the Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O site during the final stage of the gas-phase Beckmann rearrangement.
O site during the final stage of the gas-phase Beckmann rearrangement.

 Please wait while we load your content...
Please wait while we load your content...