Structural characteristics of hydrated protons in the conductive channels: effects of confinement and fluorination studied by molecular dynamics simulation†
Abstract
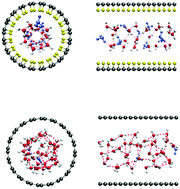
The relationship between the proton conductive channel and the hydrated proton structure is of significant importance for understanding the deformed hydrogen bonding network of the confined protons which matches the nanochannel. In general, the structure of hydrated protons in the nanochannel of the proton exchange membrane is affected by several factors. To investigate the independent effect of each factor, it is necessary to eliminate the interference of other factors. In this paper, a one-dimensional carbon nanotube decorated with fluorine was built to investigate the independent effects of nanoscale confinement and fluorination on the structural properties of hydrated protons in the nanochannel using classical molecular dynamics simulation. In order to characterize the structure of hydrated protons confined in the channel, the hydrogen bonding interaction between water and the hydrated protons has been studied according to suitable hydrogen bond criteria. The hydrogen bond criteria were proposed based on the radial distribution function, angle distribution and pair-potential energy distribution. It was found that fluorination leads to an ordered hydrogen bonding structure of the hydrated protons near the channel surface, and confinement weakens the formation of the bifurcated hydrogen bonds in the radial direction. Besides, fluorination lowers the free energy barrier of hydronium along the nanochannel, but slightly increases the barrier for water. This leads to disintegration of the sequential hydrogen bond network in the fluorinated CNTs with small size. In the fluorinated CNTs with large diameter, the lower degree of confinement produces a spiral-like sequential hydrogen bond network with few bifurcated hydrogen bonds in the central region. This structure might promote unidirectional proton transfer along the channel without random movement. This study provides the cooperative effect of confinement dimension and fluorination on the structure and hydrogen bonding of the slightly acidic water in the nanoscale channel.

 Please wait while we load your content...
Please wait while we load your content...