Modern ab initio valence bond theory calculations reveal charge shift bonding in protic ionic liquids†
Abstract
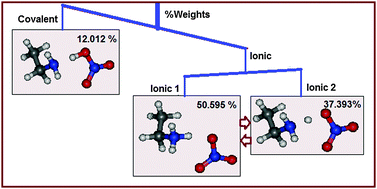
The nature of bonding interactions between the cation and the anion of an ionic liquid is at the heart of understanding ionic liquid properties. A particularly interesting case is a special class of ionic liquids known as protic ionic liquids. The extent of proton transfer in protic ionic liquids has been observed to vary according to the interacting species. Back proton transfer renders protic ionic liquids volatile and to be considered as inferior ionic liquids. We try to address this issue by employing modern ab initio valence bond theory calculations. The results indicate that the bonding in the cation and the anion of a prototypical ionic liquid, ethylammonium nitrate, is fundamentally different. It is neither characteristic of covalent/polar covalent bonding nor ionic bonding but rather charge shift bonding as a resonance hybrid of two competing ionic molecular electronic structure configurations. An investigation of other analogous protic ionic liquids reveals that this charge shift bonding seems to be a typical characteristic of protic ionic liquids while the ionic solid analogue compound ammonium nitrate has less charge shift bonding character as compared to protic ionic liquids. Further the extent of charge shift bonding character has been found to be congruent with the trends in many physicochemical properties such as melting point, conductivity, viscosity, and ionicity of the studied ionic liquids indicating that percentage charge shift character may serve as a key descriptor for large scale computational screening of ionic liquids with desired properties.

 Please wait while we load your content...
Please wait while we load your content...