Structural evidence for solvent-stabilisation by aspartic acid as a mechanism for halophilic protein stability in high salt concentrations†
Abstract
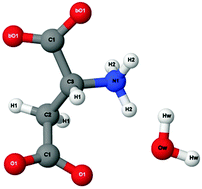
Halophilic organisms have adapted to survive in high salt environments, where mesophilic organisms would perish. One of the biggest challenges faced by halophilic proteins is the ability to maintain both the structure and function at molar concentrations of salt. A distinct adaptation of halophilic proteins, compared to mesophilic homologues, is the abundance of aspartic acid on the protein surface. Mutagenesis and crystallographic studies of halophilic proteins suggest an important role for solvent interactions with the surface aspartic acid residues. This interaction, between the regions of the acidic protein surface and the solvent, is thought to maintain a hydration layer around the protein at molar salt concentrations thereby allowing halophilic proteins to retain their functional state. Here we present neutron diffraction data of the monomeric zwitterionic form of aspartic acid solutions at physiological pH in 0.25 M and 2.5 M concentration of potassium chloride, to mimic mesophilic and halophilic-like environmental conditions. We have used isotopic substitution in combination with empirical potential structure refinement to extract atomic-scale information from the data. Our study provides structural insights that support the hypothesis that carboxyl groups on acidic residues bind water more tightly under high salt conditions, in support of the residue-ion interaction model of halophilic protein stabilisation. Furthermore our data show that in the presence of high salt the self-association between the zwitterionic form of aspartic acid molecules is reduced, suggesting a possible mechanism through which protein aggregation is prevented.

 Please wait while we load your content...
Please wait while we load your content...