Two-dimensional cyanates: stabilization through hydrogenation†
Abstract
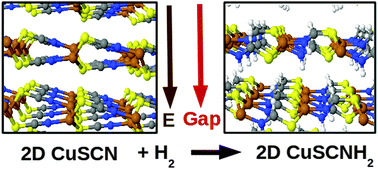
According to first-principles calculations, it should be possible to grow two-dimensional (2D) forms of copper thio-cyanate (CuSCN) and copper seleno-cyanate (CuSeCN) since their energies are only marginally higher than those of their most stable three-dimensional (3D) wurtzite structures. Here we show using the same theoretical approach that chemisorption reactions of hydrogen molecules with the above-mentioned 2D CuSCN and CuSeCN systems enhance their stability as they decrease the energy difference with respect to the corresponding hydrogenated forms of the wurtzite crystals. Hydrogenation causes a sizeable decrease in the energy band gap by 0.56 eV and 0.65 eV for hydrogenated 2D-CuSCN (CuSCNH2) and 2D-CuSeCN (CuSeCNH2), respectively. Finally, we describe the stability of hydrogen vacancies in CuSCNH2 and CuSeCNH2 and show that the presence of isolated single H vacancies or di-vacancies does not affect significantly the electronic properties of the host systems close to the valence and conduction band edges.

 Please wait while we load your content...
Please wait while we load your content...