Correlation between the acid–base properties of the La2O3 catalyst and its methane reactivity†
Abstract
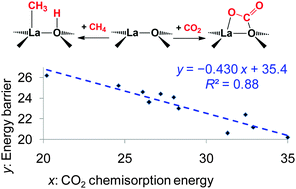
Density functional theory and coupled cluster theory calculations were carried out to study the effects of the acid–base properties of the La2O3 catalyst on its catalytic activity in the oxidative coupling of methane (OCM) reaction. The La3+–O2− pair site for CH4 activation is considered as a Lewis acid–Brönsted base pair. Using the Lewis acidity and the Brönsted basicity in the fluoride affinity and proton affinity scales as quantitative measures of the acid–base properties, the energy barrier for CH4 activation at the pair site can be linearly correlated with these acid–base properties. The pair site consisting of a strong Lewis acid La3+ site and a strong Brönsted base O2− site is the most reactive for CH4 activation. In addition, the basicity of the La2O3 catalyst was traditionally measured by temperature-programmed desorption of CO2, but the CO2 chemisorption energy is better regarded as a combined measure of the acid–base properties of the pair site. A linear relationship of superior quality was found between the energy barrier for CH4 activation and the CO2 chemisorption energy, and the pair site favorable for CO2 chemisorption is also more reactive for CH4 activation, leading to the conflicting role of the “basicity” of the La2O3 catalyst in the OCM reaction. The necessity for very high reaction temperatures in the OCM reaction is rationalized by the requirement for the recovery of the most reactive acid–base pair site, which unfortunately also reacts most readily with the byproduct CO2 to form the very stable CO32− species.

 Please wait while we load your content...
Please wait while we load your content...