The reaction mechanism for the SCR process on monomer V5+ sites and the effect of modified Brønsted acidity†
Abstract
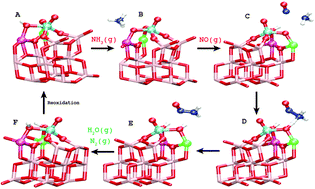
The energetics, structures and activity of a monomeric VO3H/TiO2(001) catalyst are investigated for the selective catalytic reduction (SCR) reaction by the use of density functional theory (DFT). Furthermore we study the influences of a dopant substitute in the TiO2 support and its effects on the known properties of the SCR system such as Brønsted acidity and reducibility of vanadium. We find for the reduction part of the SCR mechanism that it involves two Ti–O–V oxygen sites. One is a hydroxyl possessing Brønsted acidity which contributes to the formation of NH4+, while the other accepts a proton which charge stabilizes the reduced active site. In the reduction the proton is donated to the latter due to a reaction between NH3 and NO that forms a H2NNO molecule which decomposes into N2(g) and H2O(g). A dopant substitution of 10 different dopants: Si, Ge, Se, Zr, Sn, Te, Hf, V, Mo and W at each of the sites, which participate in the reaction, modifies the energetics and therefore the SCR activity. We find that Brønsted acidity is a descriptor for the SCR activity at low temperatures. Based on this descriptor we find that Zr, Hf and Sn have a positive effect as they decrease the activation energy for the SCR reaction.

 Please wait while we load your content...
Please wait while we load your content...