SANS study on the solvated structure and molecular interactions of a thermo-responsive polymer in a room temperature ionic liquid†
Abstract
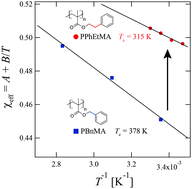
We have utilized small-angle neutron scattering (SANS) to quantitatively characterize the LCST-type phase behavior of the poly(benzyl methacrylate) (PBnMA) derivative poly(2-phenylethyl methacrylate) (PPhEtMA) in the deuterated ionic liquid (IL) d8-1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)amide (d8-[C2mIm+][TFSA−]). The SANS curves showed a discontinuous change from those characteristics of dispersed polymer chains to those of large aggregates of PPhEtMA chains suspended in the IL solution, indicating that phase separation occurs discontinuously at Tc. Furthermore, we evaluated the enthalpic and entropic contributions to the effective interaction parameter χeff of PPhEtMA in [C2mIm+][TFSA−] and compared them with those of PBnMA. The absolute value of the enthalpic contribution observed for PPhEtMA was smaller than that for PBnMA. This difference in the enthalpic term can be attributed to the unfavorable interaction between the IL and the alkyl group in the side chain of PPhEtMA. In addition, the temperature dependence of χeff was smaller than the previously reported value for a thermo-responsive polymer in an aqueous system. It was found out that the strong dependence of Tc on the chemical structure in IL systems originated from the relatively smaller temperature dependence of χeff.

 Please wait while we load your content...
Please wait while we load your content...