Dissociative electron transfer in polychlorinated aromatics. Reduction potentials from convolution analysis and quantum chemical calculations†
Abstract
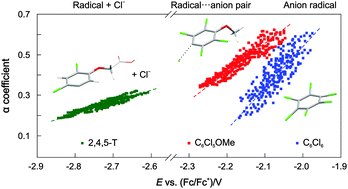
Formal potentials of the first reduction leading to dechlorination in dimethylformamide were obtained from convolution analysis of voltammetric data and confirmed by quantum chemical calculations for a series of polychlorinated benzenes: hexachlorobenzene (−2.02 V vs. Fc+/Fc), pentachloroanisole (−2.14 V), and 2,4-dichlorophenoxy- and 2,4,5-trichlorophenoxyacetic acids (−2.35 V and −2.34 V, respectively). The key parameters required to calculate the reduction potential, electron affinity and/or C–Cl bond dissociation energy, were computed at both DFT-D and CCSD(T)-F12 levels. Comparison of the obtained gas-phase energies and redox potentials with experiment enabled us to verify the relative energetics and the performance of various implicit solvent models. Good agreement with the experiment was achieved for redox potentials computed at the DFT-D level, but only for the stepwise mechanism owing to the error compensation. For the concerted electron transfer/C–Cl bond cleavage process, the application of a high level coupled cluster method is required. Quantum chemical calculations have also demonstrated the significant role of the π*ring and σ*C–Cl orbital mixing. It brings about the stabilisation of the non-planar, C2v-symmetric C6Cl6˙− radical anion, explains the experimentally observed low energy barrier and the transfer coefficient close to 0.5 for C6Cl5OCH3 in an electron transfer process followed by immediate C–Cl bond cleavage in solution, and an increase in the probability of dechlorination of di- and trichlorophenoxyacetic acids due to substantial population of the vibrational excited states corresponding to the out-of-plane C–Cl bending at ambient temperatures.

 Please wait while we load your content...
Please wait while we load your content...