Optical properties of the hydrated charged silver tetramer and silver hexamer encapsulated inside the sodalite cavity of an LTA-type zeolite†
Abstract
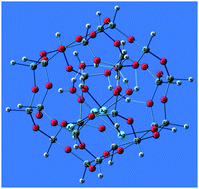
The optical spectra in the UV-VIS region of the hydrated doubly charged tetramer Ag42+ and hydrated multiply charged hexamer Ag6p+ silver clusters encapsulated inside the sodalite cavity of an LTA-type zeolite have been systematically predicted using DFT, TD-DFT and CASSCF/CASPT2 methods. The optical behaviour of the model hydrated clusters [Ag6(H2O)8(Si24H24O36)]p+ is very sensitive to their charge. Among the cations [Ag6(H2O)8(Si24H24O36)]p+, only the embedded hydrated quadruply charged silver hexamer [Ag6(H2O)8(Si24H24O36)]4+ shows a strong absorption band at ∼420 nm (blue light) and emits light in red color. The absorption spectrum of the hydrated doubly charged silver tetramer cluster [Ag4(H2O)m(Si24H24O36)]2+, which shifts slightly and steadily with the increasing amount of interacting water molecules to longer wavelengths, has a strong peak in the blue region. The water environment forces the silver tetramer to relocate into one side of the cavity instead of at its center as in the case of the non-hydrated [Ag4(Si24H24O36)]2+ cluster. Water molecules act as ligands significantly splitting the energy levels of excited states of the Ag42+ and Ag64+ clusters. This causes the absorption spectra of the clusters to broaden and the emission to shift to the green-yellow and red part of the visible region.

 Please wait while we load your content...
Please wait while we load your content...