Structural and surface coverage effects on CO oxidation reaction over carbon-supported Pt nanoparticles studied by quadrupole mass spectrometry and diffuse reflectance FTIR spectroscopy
Abstract
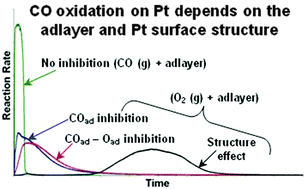
The CO oxidation reaction on carbon-supported Pt nanoparticles (average size of 2.8 to 7.7 nm) was studied under flowing conditions at atmospheric pressure and temperatures between 300 and 353 K by coupling quadrupole mass spectrometry (QMS) and diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS). The Pt loading was varied between 20 and 60 wt%. Gases diluted in He (0.5 mol%) were used together with Ar as a tracer. Reactions with CO and O2 introduced separately onto the samples were studied by QMS, applying successive step changes of the reaction mixtures. Variations in the rate of the reactions were observed and correlated with changes of the calculated coverage of the Pt surface by CO and/or O adspecies at varying steps of the experiment. The transient reaction of CO(g) with adsorbed O (Oad) was fast and mass transport-limited while that of O2(g) with adsorbed CO (COad) was sluggish. Following the same experimental procedures, FTIR spectra of adsorbed CO after varying steps were recorded, confirming the variations of COad and Oad as determined by QMS and indicating changes in the CO distribution over varying types of Pt surface sites. The influence of the adlayer composition (co-adsorption of COad and Oad), the particle size/structure and some possible surface reconstruction effects on the CO oxidation rate were evidenced and discussed. The structure of the Pt nanoparticles supported on carbon appears as an important factor for the efficiency of the so-called O2 bleeding as a CO mitigation strategy in polymer electrolyte membrane fuel cells.


 Please wait while we load your content...
Please wait while we load your content...