On the absolute photoionization cross section and dissociative photoionization of cyclopropenylidene†
Abstract
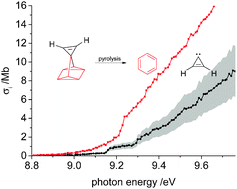
We report the determination of the absolute photoionization cross section of cyclopropenylidene, c-C3H2, and the heat of formation of the C3H radical and ion derived by the dissociative ionization of the carbene. Vacuum ultraviolet (VUV) synchrotron radiation as provided by the Swiss Light Source and imaging photoelectron photoion coincidence (iPEPICO) were employed. Cyclopropenylidene was generated by pyrolysis of a quadricyclane precursor in a 1 : 1 ratio with benzene, which enabled us to derive the carbene’s near threshold absolute photoionization cross section from the photoionization yield of the two pyrolysis products and the known cross section of benzene. The cross section at 9.5 eV, for example, was determined to be 4.5 ± 1.4 Mb. Upon dissociative ionization the carbene decomposes by hydrogen atom loss to the linear isomer of C3H+. The appearance energy for this process was determined to be AE0K(c-C3H2; l-C3H+) = 13.67 ± 0.10 eV. The heat of formation of neutral and cationic C3H was derived from this value via a thermochemical cycle as ΔfH0K(C3H) = 725 ± 25 kJ mol−1 and ΔfH0K(C3H+) = 1604 ± 19 kJ mol−1, using a previously reported ionization energy of C3H.

 Please wait while we load your content...
Please wait while we load your content...